Optimizing Affiliate Engagement is a Key Step for the Last Mile
By Katherine Yang-Iott
You may have heard of the saying to ‘treat feedback as a gift’. If so, then our latest study feels like a gift that keeps on giving. Our 2023 Optimizing Affiliate Engagement to Improve Performance of Key Regulatory Activities survey is a landmark study designed to capture the voice of the affiliate. As most life science companies want to be better and get better at managing key regulatory information on a truly global scale, improving how affiliates work and how headquarters interacts with and supports those affiliates are critical components.
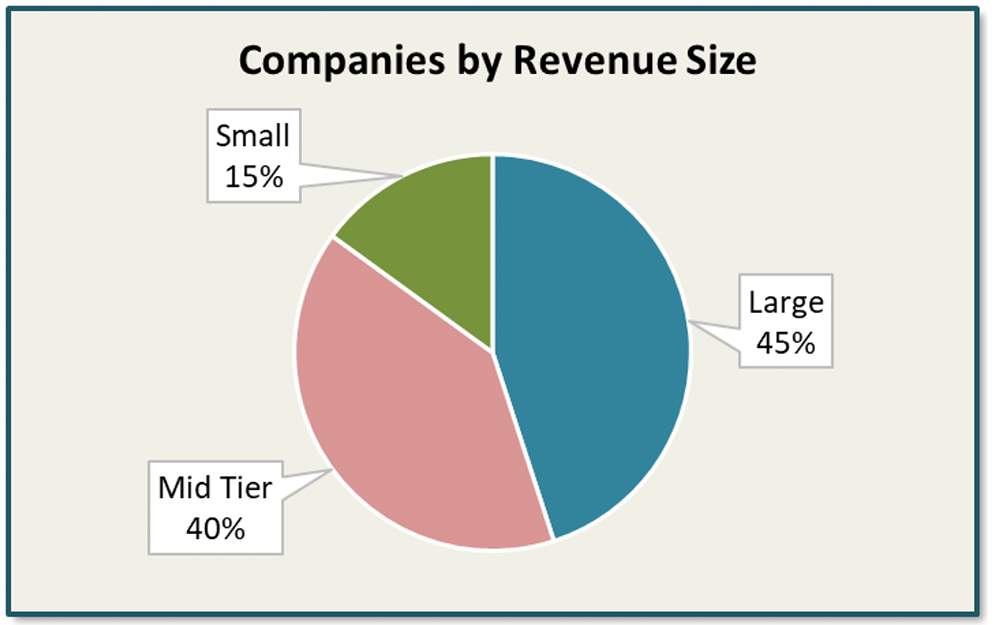
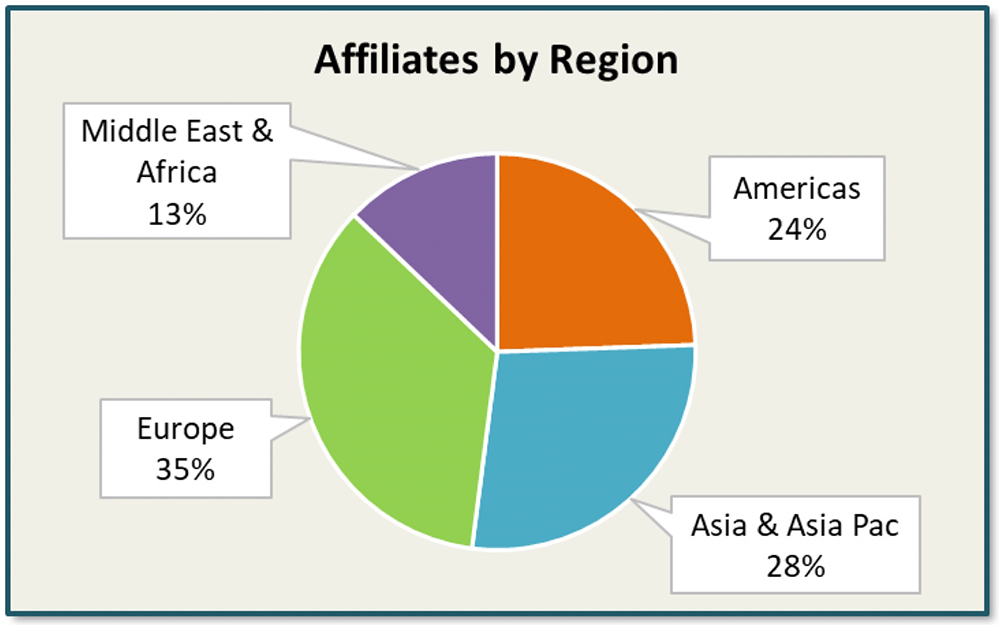
In our research, we had 320 affiliates participate from 94 countries. The affiliates represented 20 unique companies across the biopharmaceutical and medtech landscape.
- How affiliates work – what global systems and local tools do they use and how much time are they spending on managing key regulatory activities such as registrations, label management, and health authority interactions?
- How are affiliates involved with process design, resourcing estimates, and system configuration decisions?
- Affiliate’s perspective – process efficiencies of key regulatory activities, data quality levels in key systems, impact of technology and system investments for Global E2E RIM.
- Affiliate engagement with headquarters – understanding key barriers for collaboration and affiliate involvement with regulatory decisions and improvements.
What we found was interesting, although there is progress in terms of affiliates using global systems more often (increase from 13% in 2015 to 48% in our current study), they continue to spend a significant amount of time in local and regional tools to manage all requirements. Over 50% of affiliates also expressed their desire to be more involved with activities such as resource planning, process design, and system enhancement decisions (governance). Ultimately, as the global regulatory excellence journey continues, what needs to happen involves part effort from headquarters, the affiliate offices, and even the solution providers. Global system usability and tailored training needs to be improved at the local level as most users are “infrequent” and may only use the global systems several times a month. Headquarters need to better utilize regional structures to enhance communication to the affiliate level and also have a balanced representation of different affiliates within their company to provide valuable feedback on process and system design. Too often affiliate representations come from larger affiliates as they have more resource flexibility – this biases the affiliate view as most offices have under 4 team members and are not well represented in improvement initiatives.
Even solution providers can deepen their knowledge of a ‘day in the life of a local affiliate’ to better design solutions by understanding the depth of regional and size of affiliate nuances. This group effort is the key to solving that ‘last mile’.
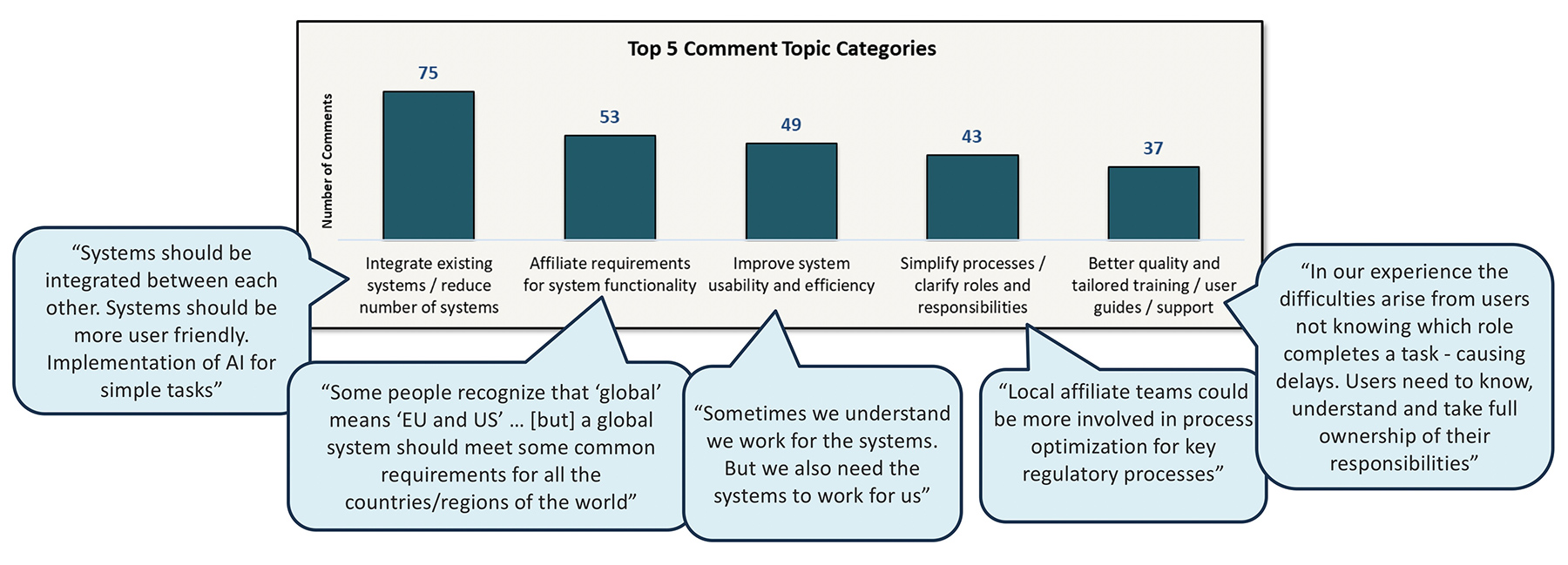
I started out this post by writing about feedback. Our last question in our study asked affiliates to share with us what “the one thing their company could do” in terms of how regulatory information is managed that would help them improve performance at their affiliate. We received 266 affiliate office responses with the overarching theme of “simplification and connected systems”. Although feedback doesn’t always result in change, it’s hard to change in the right direction without it.
If you would like to hear more about the study results, check out our podcast series. We have one highlighting the initial key learnings, one where we talk about how to use the study report and apply the learnings, and even one digging in to what the study finds for device companies. Our white paper will be published later this winter.