Making the Case for a Continuous Improvement Programme
By Steve Gens
Our latest research demonstrates that a continuous improvement programme (CIP) has a measurable effect on your regulatory information management (RIM) programme performance over time.
During a recent client engagement that required additional analysis from our 2018 World Class RIM research, we made some interesting and pertinent discoveries about CIP with performance measures, specially companies having this combination outperformed those without a CIP in four areas.
We carried out this analysis for an EU based life sciences company who had some interesting ratings from our World Class RIM benchmark. They rated higher than most in the number and type of performance metrics but very low in the overall efficiency levels. We typically see a correlation between strong efficiency of the 18 RIM capability categories and having both cycle time and quality metrics (see the listing in our recent RIM Whitepaper ). In this case, they were tracking and measuring many of the right metrics, but did not have the organizational strategy and processes of a CIP where the data can dictate when valuable improvements lie. One of our recommendation was putting a CIP in place.
Support for CIP
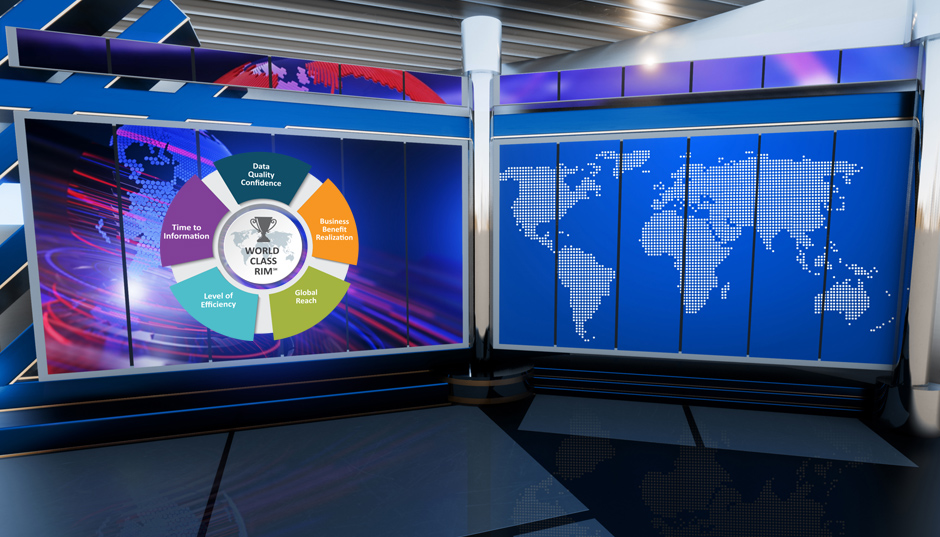
So, how did we determine the value of CIP? First, we carried out an analysis of companies with a CIP based on the data from our 2018 World Class RIM: Connections to QMS, Process Change Control, and Supply Release of 70 companies. Thirty companies have a CIP and forty did not. Now there were some unknowns – such as whether the CIP programme was mature or newly implemented and how regular were the performance metrics collected. We compared the average RIM efficiency, business benefit realisation, time to report information, and data quality of the companies with CIP to those without a CIP.
What this showed was those with a CIP were 20% higher in terms of average capability efficiency, and 26% more efficient with regards to achieving business benefits, which is really what matters most to businesses. In addition, these companies can report information quicker (17% difference) and their confidence levels in data quality were higher (16%).
These are significant findings and show that investing in CIP is important. Many times, organisations look at “episodic” or project-based initiatives to improve performance only. These may happen every 5 – 7 years and are important, however having both a CIP and project-based change is the right combination, in our opinion. CIP may result in three or four smaller changes a year (e.g. process or data quality improvements), but over time, the benefit is significant.
A successful CIP is more than just a programme; rather, it’s an organisational strategy. That doesn’t mean it has to be vast. It could just involve a couple of people who are dedicated to the CIP. But it’s important to think across the organisation and not limit these RIM improvements to regulatory. By achieving those improved processes, better data quality, and enhanced metrics at the connection points between regulatory as well – supply-release, commercial, clinical, safety, etc. – you’re starting to achieve continuous improvement across the business.