Regulatory Use of Advanced Technology
By Katherine Yang-Iott and Greg Brolund
In an ideal state, Regulatory’s use and adoption of advanced technology would be swift, far reaching, and effective. But this is easier said than done. Managing regulatory information is highly complex, in part, because requirements vary and often change across geographic locations. Traditional methods of managing regulatory information are often rigid and bound by controlled systems that rely heavily on manual processes with specialized experts needed to use and manage them. All that being said, these are the same arguments as to why industry should move to embrace the use of advanced technologies.
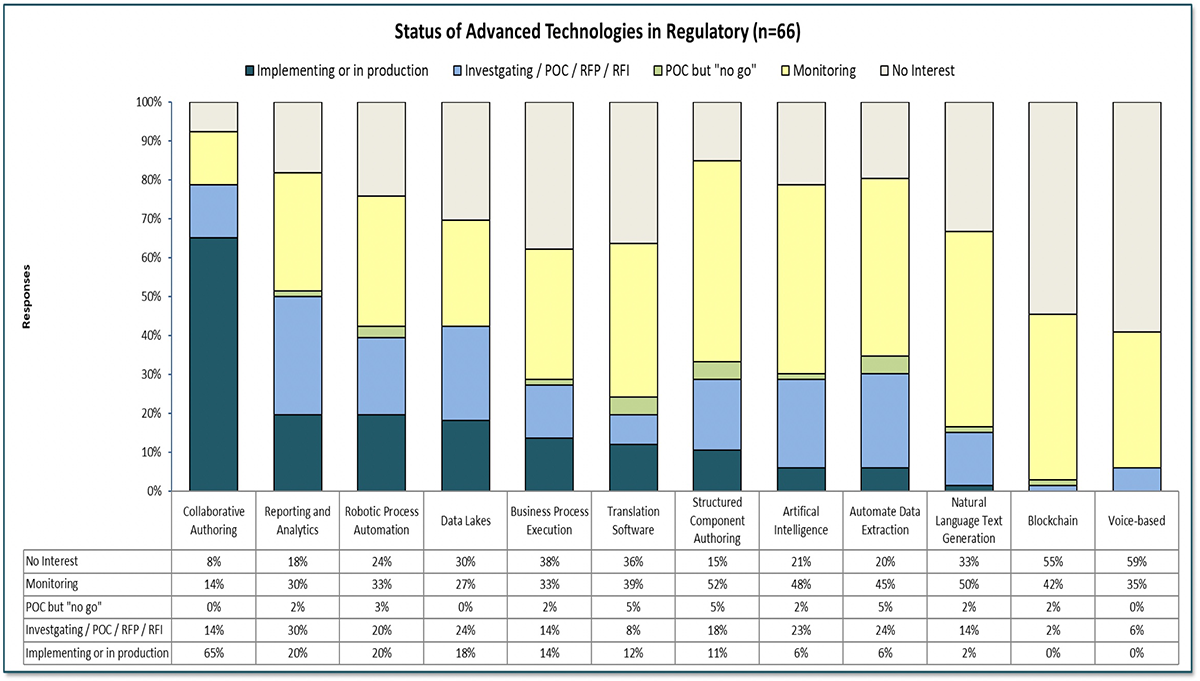
Our 2020 World Class Regulatory Information Management (RIM)℠ Survey research included an examination of the current state of technology usage in Regulatory along with the areas of opportunity and interests for regulatory experts. We asked our participants if they were using or trying to use any of the following technologies within Regulatory.
The results (see figure below) show that, although adoption is relatively low for all technologies except collaborative authoring, more than 25% of the companies expressed significant interest in most of the listed technologies. Perhaps disappointing to some is that the highest adoption was collaborative authoring, which few would consider an advanced technology. “Improved productivity or efficiency” and “Improved user satisfaction” where the two most often cited benefits achieved by companies with any of these in use for at least 6 months.
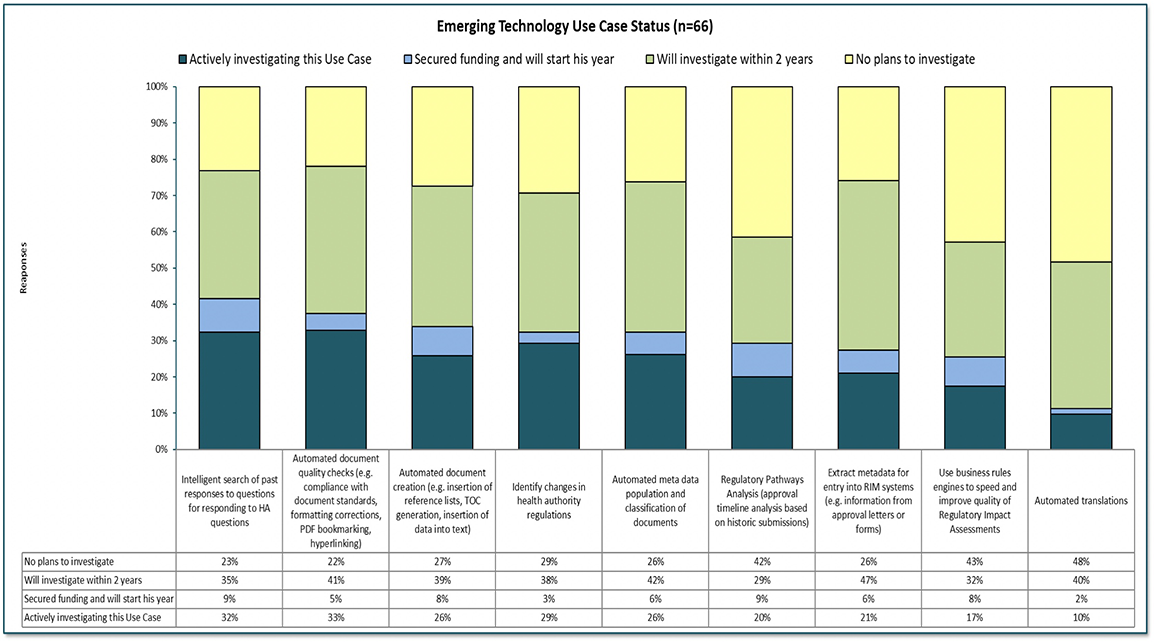
The survey also asked respondents to share what emerging technologies they were currently experimenting with or planning to investigate, in terms of use cases. In the graph below, our data provides some insight as to where companies see opportunities for automation. Most of these use cases are dependent on AI / Natural Language Processing capabilities which are likely to continue to mature over the next few years. 33% of survey participants are actively investigating automated document quality checks, 32% intelligent search of past responses to Health Authority responses, and 29% identify changes in health authority regulations. Large companies have an active interest in most of these use cases, whereas most mid-size and small companies are only planning to investigate within 2 years.
When it comes to adopting advanced technologies, consider that many regulatory activities require significant manual effort. For example, Roche is using the principle for Robotic Process Automation (RPA) that “if you can do it by hand, you can automate it” and that RPA can be built into existing RIM applications. The promise of data automation can significantly reduce the amount of lower value work, such as data entry or identifying emails that should be addressed by regulatory so that regulatory experts can spend more time on more value added work, especially since Regulatory’s capabilities to identify and mitigate potential compliance risks need to become more sophisticated as regulatory requirements become more complex.
Another important technological impact is it can increase productivity by reducing complexity. Deploying these new technologies can benefit both the foundational functions (i.e. improving data quality to promote high data confidence and real time monitoring) as well as more advanced functions of regulatory needs (i.e. predictive analytics). Advanced reporting and analytics can identify and report past occurrences while building in real time prompts and checks to maintain high quality data. Currently, many of the use cases already implemented support these types of reactive activities. AI and robotics can go a step further by anticipating what could go wrong and prevent those events from happening in the future. Automation first, intelligence next, and finally advanced analytics.
We will continue to track and report on the progress of advanced technology use cases. This year, we have gained significant insights from our group of high performers as measured by the Gens and Associates World Class RIM standard. Moving forward, as companies share their experience with technology advancements, we will continue to gain insights on the benefits realized that can encourage wider adoption of these technologies across industry and make these advancements the rule rather than the exception. Our data suggests that the adoption of advanced technology use is still in the ‘early days’ but promises to improve efficiency and performance without necessarily increasing regulatory head count. Time will show how using advanced technologies to drive efficiency and improve quality is an effective strategy for regulatory organizations to invest in.
1 Roche presentation to IRISS Forum, February 2020