Quality: What’s at stake?
By Katherine Yang-Iott
Surviving a pandemic year, especially in the life sciences industry, means that we all had some rude awakenings. But that’s not necessarily a bad thing. Our 2020 Covid-19 study tells us that crisis often forces us to roll up our sleeves to get things done, often with some new found ingenuity and the realization that a better way might exist. With the speed and innovation in which effective vaccines were developed and approved to address the pandemic, a bigger “Quality” conversation developed surrounding public health and safety.
In early April, it was reported that 15 million COVID19 vaccine doses were destroyed as it did not meet quality standards due to manufacturing plant contamination at a specific site. While the Quality System worked in this instance and found the error prior to product distribution, these types of issues are not uncommon and yet it should be. What investigators and quality evaluators found in this particular case was that the site fell short on following basic industry standards. These types of common offenses and findings have led experts to wonder about the prevalence of deeper quality issues and the overall performance of companies’ quality management systems.
Here at Gens & Associates, we just launched our 37th Life Science study exploring the multiple dimensions of quality. Our Quality Benchmark: Enhancing Quality beyond Compliance study aims to establish the initial baseline reading of where industry is currently at with their Quality systems and practices. How are companies thinking about Quality and where does it need to go in order to meet and exceed the needs and demands of their company, the authorities and most importantly, the patients. Our benchmarking work is known for the insights plus the precision by providing a unique perspective on industry status, peer comparison, challenges and opportunities, investment priorities, and the solution provider landscape. If you want to find out how your company stacks up against your peers, enroll in our study. An additional benefit for this study is how companies will be able to compare their quality performance internally across the different areas of quality, a unique output from this research due to the design of the survey.
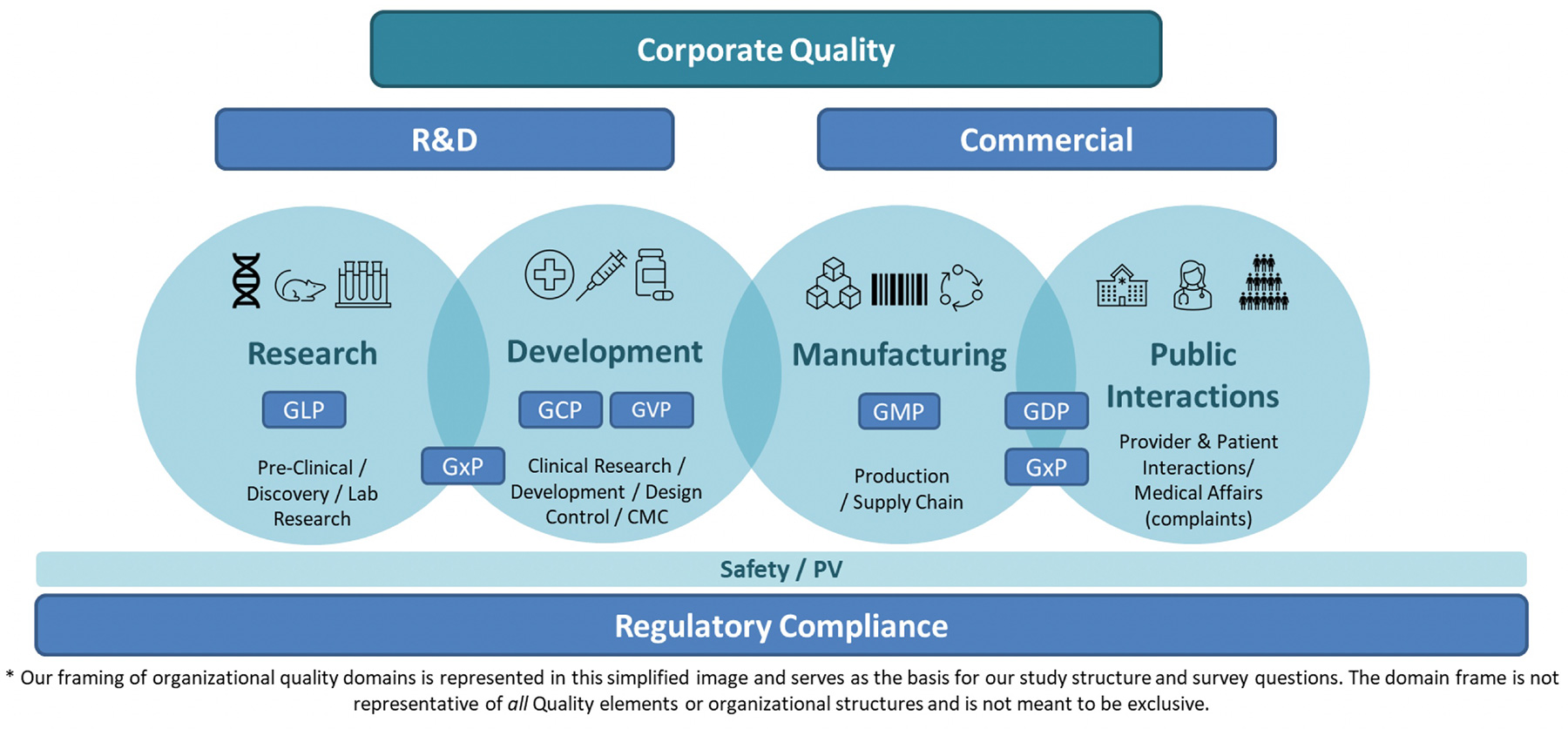
We have specific sections of the survey focusing on 4 main Quality Domains:
During 2016-2019, St. Gallen did some extensive Quality metrics research with the FDA. Their summary of quality excellence is described as, “…an advanced approach to quality which goes beyond merely being compliant with regulations. Quality excellence is patient-driven, culturally embedded and built into the processes and behavior of an organization”. Culture has always been a keystone in all of our research and we certainly believe a culture of quality is the ideal environment to embrace and aspire towards. The design of our study allows companies to use the results like a diagnostic tool, to see where they fall on the culture of quality continuum. Below is an image of that continuum and the data from the study can help companies identify whether they embody characteristics more as a reactive ‘Quality by inspection’ type of company or have they incorporated practices embracing more of the ‘Quality by Design’ characteristics of the 3 P’s principles (proactive, predictive, preventative quality), evolving closer to the ideal Culture of Quality environment.
We collaborated with 16 sponsors via design sessions and 9 design partners (Quality experts, consultancies, and solution providers) to create this first G&A Quality benchmark. One of our partners, KPMG, published an article envisioning the potentials of Quality in 2030. They recognized how much transformation has already started for Quality in response to, “…more stringent regulatory environment, new delivery mechanisms, and increased supply chain complexity. The need for further change will only be amplified by emerging trends, such as digitalization, new business models, and disruptive competitors”. In thinking about these macrotrends and where quality is headed, our research and our findings can either be confirming or reveal new stepping stones towards that future-ready Quality organization.
Ultimately, patient safety is everybody’s top priority. Regardless of a global health crisis or not, people should never have to be wary of using regulated medicinal products, worry about the safety or quality of what they are receiving. As the developers and the distributors of medicinal products, what’s at stake with quality is not just your company’s reputation or financial risk, what’s at stake is the greater good of global public health.