IDMP/SPOR: It’s time to talk about “Submission-Ready Data”
By Kelly Hnat
The long-awaited IDMP/SPOR Version 2 EU Implementation Guide was published in February this year, and if my inbox is any indication, companies all over industry are diving into implementation mode. That old urgency we felt in 2015/2016 is coming back, although this time around it seems to me that, as an industry, we are a bit less frightened and a bit more educated. But understanding conceptually what IDMP is, what SPOR is doing, and what the agency requirements are does not make this implementation easy, or even straightforward. Done right, SPOR preparedness is a big deal, like building a house. Done poorly, it’s still a big deal – but more like a natural disaster that might drown our Regulatory Operations teams. In the same way that the introduction of eCTD changed the way our submission documents were authored, leading us all to learn the phrase ‘submission-ready documents,’ I believe this new era of structured data submissions should have us all thinking about, and preparing for, ‘submission-ready data.’
Why Submission-Ready Data?
In our 2020 World-Class RIM survey we asked companies what strategies they plan to employ as they implement solutions to support structured data submissions like IDMP/SPOR. The overwhelming majority of our respondents (69%) reported that they plan to implement processes and systems to enable automatic generation of structured data submissions from their systems. To generate these submissions automatically, this data will need to be accurate, timely, and in the correct format: that is, ‘Submission Ready.’ Submission-Ready Data is data we are happy to have an assessor review as a representation of the registration we are seeking to establish or modify. It is data generated by processes that we trust will generate correct information, held in systems we trust will serve up the right values when we generate the FHIR message for SPOR PMS, or in some future Health Authority requirement.
How do we get there?
Achieving this level of trust will take some work. In the first place, we are changing the purpose of our RIM systems, which for most companies will be the source for generating their IDMP/SPOR submissions. Although there are a few companies that do leverage data in their RIM systems for critical purposes such as supporting compliant release of product to markets, never before have we assigned to our RIM systems the role we are asking them to play now. We are not just expecting them to be the authoritative source for structured data representation of our approved registrations; we are asking them to become the authoritative source for structured data representations of our proposed registrations. And this is where it gets complicated.
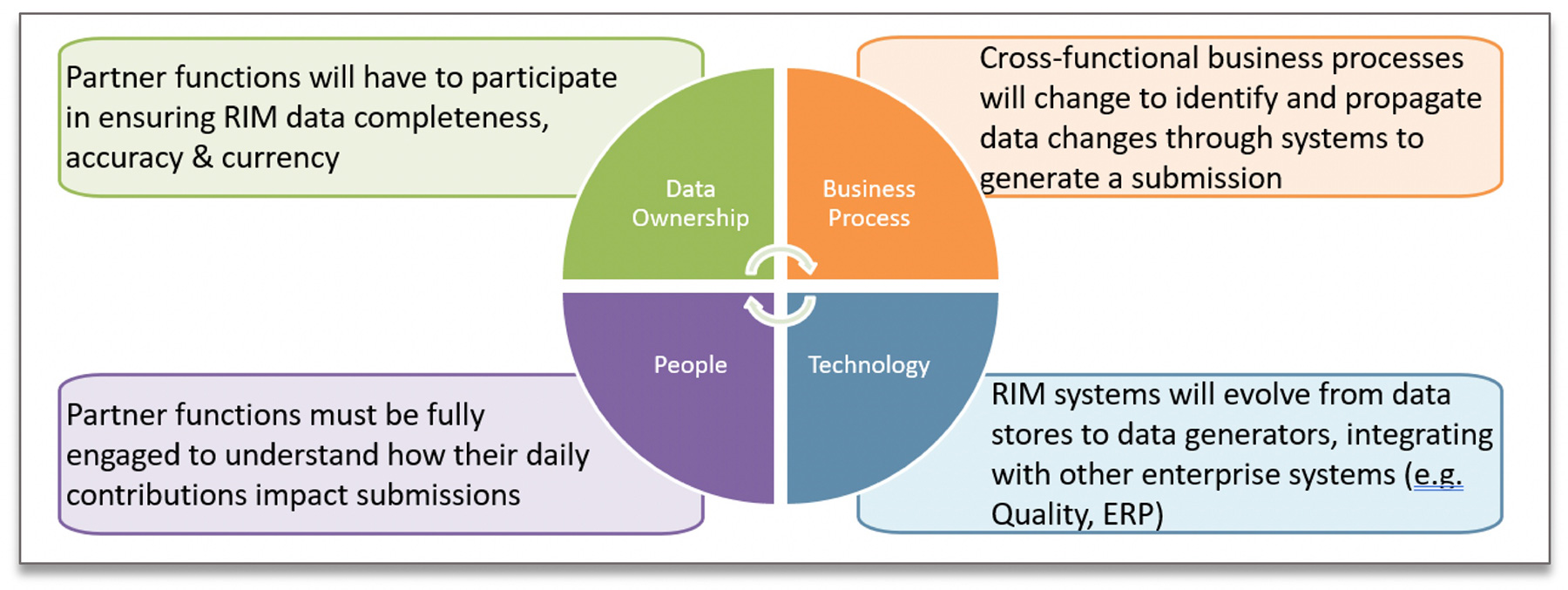
To achieve Submission Ready Data, industry initiatives for IDMP/SPOR will need to look at the problem, and its solution, from several perspectives: Data Ownership & Governance, Business Process, People, and Technology, as shown in the figure below.
Each of these components will play an important role in the overall success of a company’s implementation. The crux of the challenge for IDMP/SPOR preparation is that we are primarily concerned with proposed values for our registrations, rather than the approved values. This means Regulatory Affairs must work closely with the upstream functions responsible for generating changes to the currently approved values to clearly define the data ownership and accountability while strengthening and expanding existing variation management processes to ensure the necessary data changes are communicated or propagated effectively and accurately. There is a critical question that every component of your solution should seek to answer: How will you know it is the right data, with the right value, for this particular submission?
In-process Data Management
The EMA IDMP/SPOR Target Operating Model (TOM) process brings review and quality control of structured product data submissions into the existing regulatory assessment process. The TOM will result in high quality, mastered data within the EMA’s PMS database using ‘in-process’ data management – building quality into the existing business process. Fortunately for industry, many RIM software providers are also building ‘in-process’ data management for IDMP/SPOR data into their systems, as we are learning during our latest research project, the Gens & Associates IDMP Provider Capability & Readiness Assessment. By managing the required data as part of the normal business flows of the RIM system and providing the ability to control the application of new data values by tracking them through an initial MA or variation lifecycle, RIM systems can support the more controlled processes necessary to achieve submission readiness for our structured data.
You can learn more about which vendors are supporting ‘in-process’ IDMP data management, as well as what other capabilities are being developed to prepare for IDMP requirements, in the upcoming IDMP Provider Capability & Readiness Assessment report, led by me and Greg Brolund. The report is expected to be published in mid-July and you can find more information about it here.