Preparing for Data Driven Submissions
By Kelly Hnat
Almost exactly a year ago, EMA announced a U-turn on its IDMP/SPOR implementation plans: they set aside the expected requirement to prepare FHIR messages to accompany eCTD submissions in favor of a plan to collect structured data using the planned DADI web-based electronic application forms. Just last week that new DADI form went live for optional use in the new Product Lifecycle Management (PLM) Portal, though it does not yet capture the detailed structured data that was originally anticipated when EMA made its November 2021 announcements. Including structured data changes in DADI is in scope for planned releases late next year. Still unknown is what requirements EMA may plan for the correction and enhancement of migrated xEVMPD data in the PMS database; in fact, EMA has not yet completely migrated the non-CAP data into PMS from the xEVMPD database (they do expect to complete this early next year).
EMA’s plans and timelines have changed, but preparing for a structured data submissions future rightly continues to be a priority for regulatory organizations. While the EMA implementation has had its hiccups, global regulator and industry interest in a structured data future for medicinal product information cannot be denied. Initiatives and consortiums like ePI, UNICOM, the Global IDMP Working Group, Accumulus, PQ/CMC, and Gravitate Health, are proliferating, and the scales are pretty well tipped towards a structured data future.
What should sponsors be doing to best position themselves for the day when the promise of structured data submissions becomes real? Preparing for data-driven submissions is primarily a task of ensuring the data that could become part of a future submission is being created and maintained in a submission-ready manner. We believe a critical first step is to begin to treat regulatory structured data as a true asset, and to establish data quality and data governance structures and practices to lay the foundation for wherever the structured data future may lead. Establishing capabilities such as a Regulatory Data Quality Office or Council, and roles like a designated Data Quality Officer and Data Stewards can support formalizing quality practices and establish processes for planning quality into the regulatory processes that touch data. These roles and structures should also focus attention on mastering reference data, which is a critical step to preparation for SPOR as well as providing a framework for future requirements.
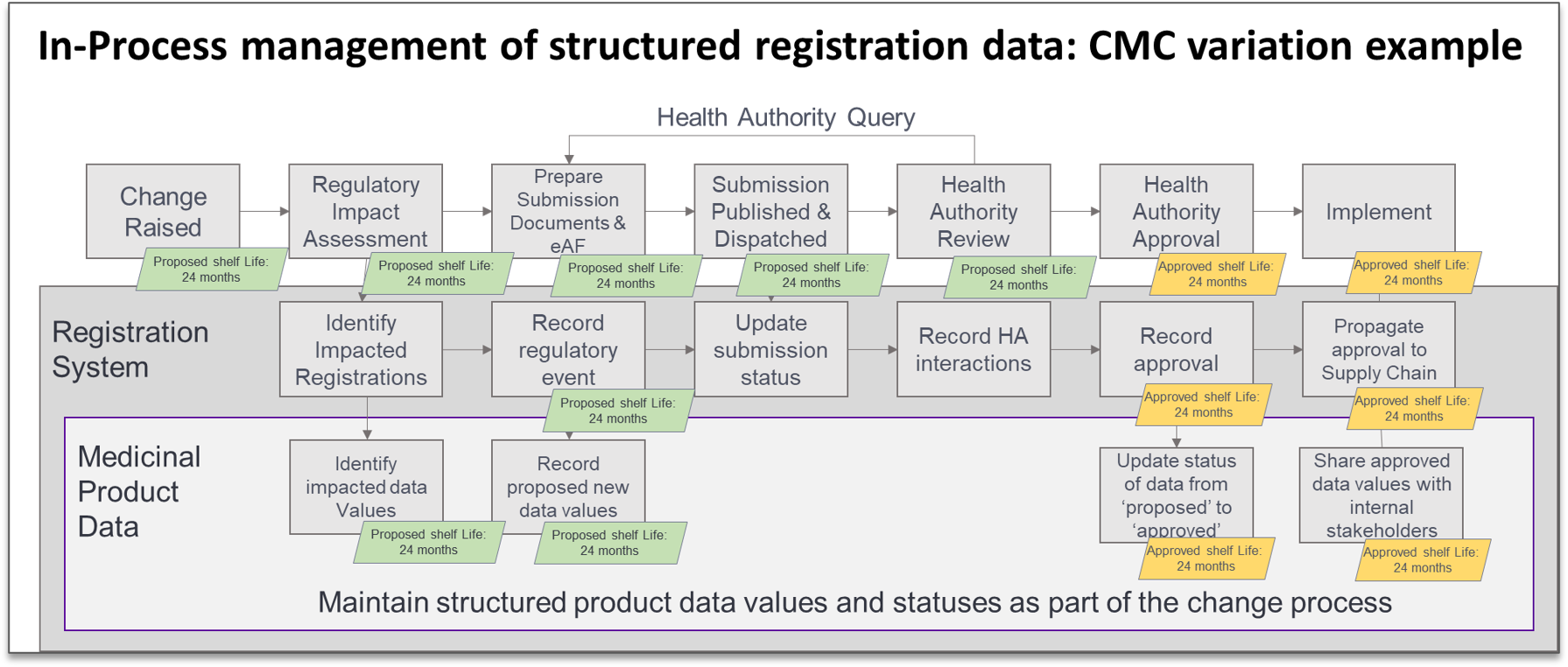
We also believe that thoughtfully integrating the creation and maintenance of critical structured regulatory data into day-to-day regulatory business processes provides a path to sustainable data quality. For example, align the tasks of capturing structured representation of data changes in the registration system with the work of preparing the dossier components in order to establish ‘submission-ready data’ in the registration systems (see an example in the figure above). This approach, built on a clear foundation of accountability and data ownership, shifts the focus and importance of what were formerly considered administrative tasks when we make clear that these data values could be provided in structured form to a health authority at any time. Education and awareness are critical to success here – many of the original sources of the information may not understand that this data could eventually wind up in a submission. Building a broad understanding of the new scope of critical data elements will help in gaining support and achieving higher data quality.