Regulatory Intelligence Automation – A Challenging Endeavor
By Greg Brolund
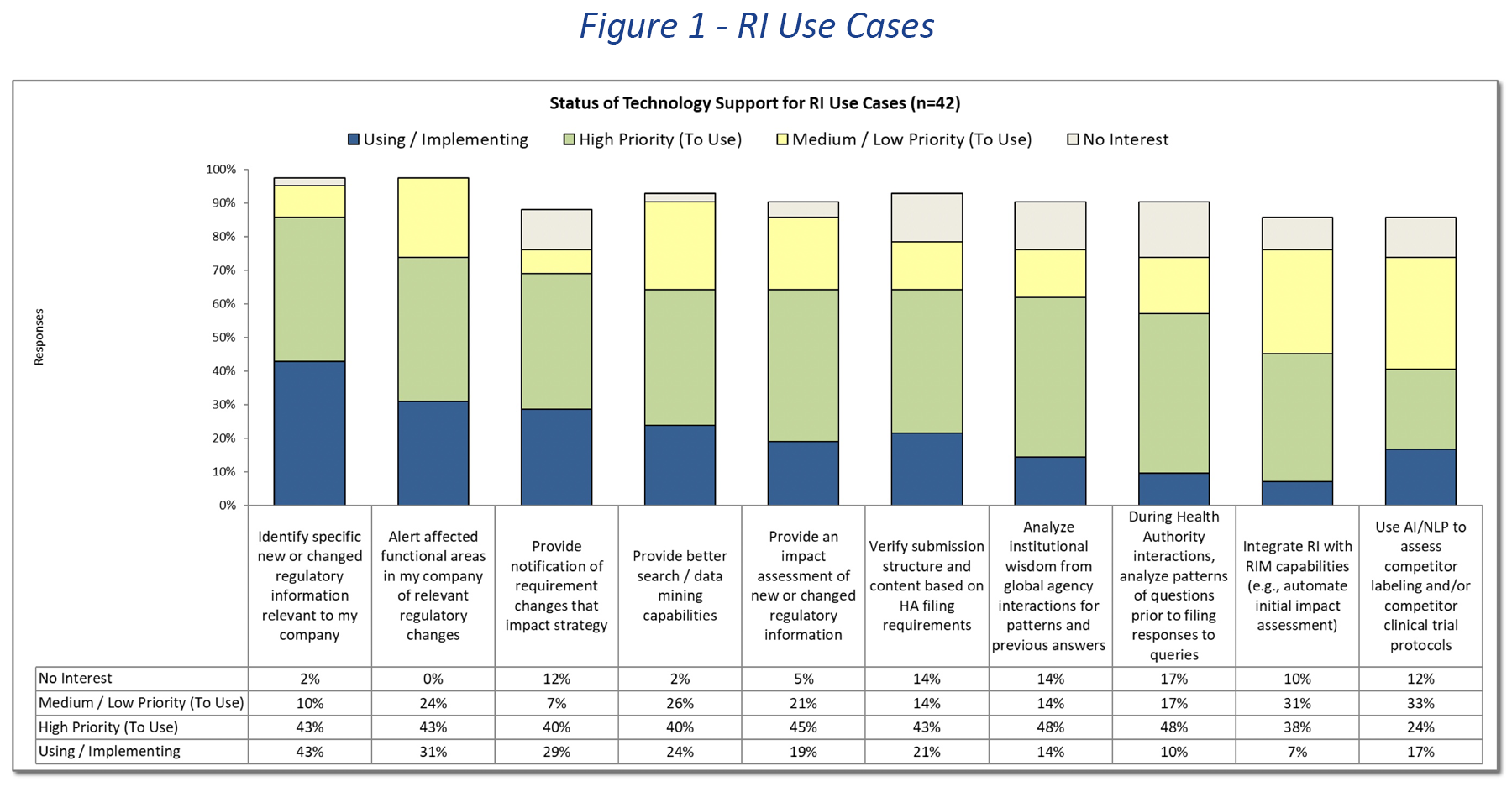
Managing regulatory intelligence information and delivering the broad variety of services expected of regulatory intelligence departments continues to be a challenge for the biopharmaceutical industry. The Gens & Associates 2022 Regulatory Intelligence Industry Survey (n = 42) identified the degree of technology support for ten specific use cases. The survey also explored the level of automation being applied to core activities carried out in regulatory intelligence programs.
In Figure 1, ten use cases are shown with the level of technology support currently in place or where there is a priority assigned to providing technology support. Technology support is broadly defined and ranges from provision of a structured database of regulatory requirements through early efforts to apply natural language processing and artificial intelligence analysis to databases and documents. It is reasonable to assume that 6 of the 10 use cases would require intelligent analysis of internal and external information from multiple sources. These sources range from structured databases (e.g., country filing requirements) to unstructured documents including presentations, working group notes and specific experiences documented in a variety of file formats. The primary challenge for these use cases is the availability of complete, authoritative and reliable structured and unstructured information. Without authoritative sources, technology solutions are ineffective.
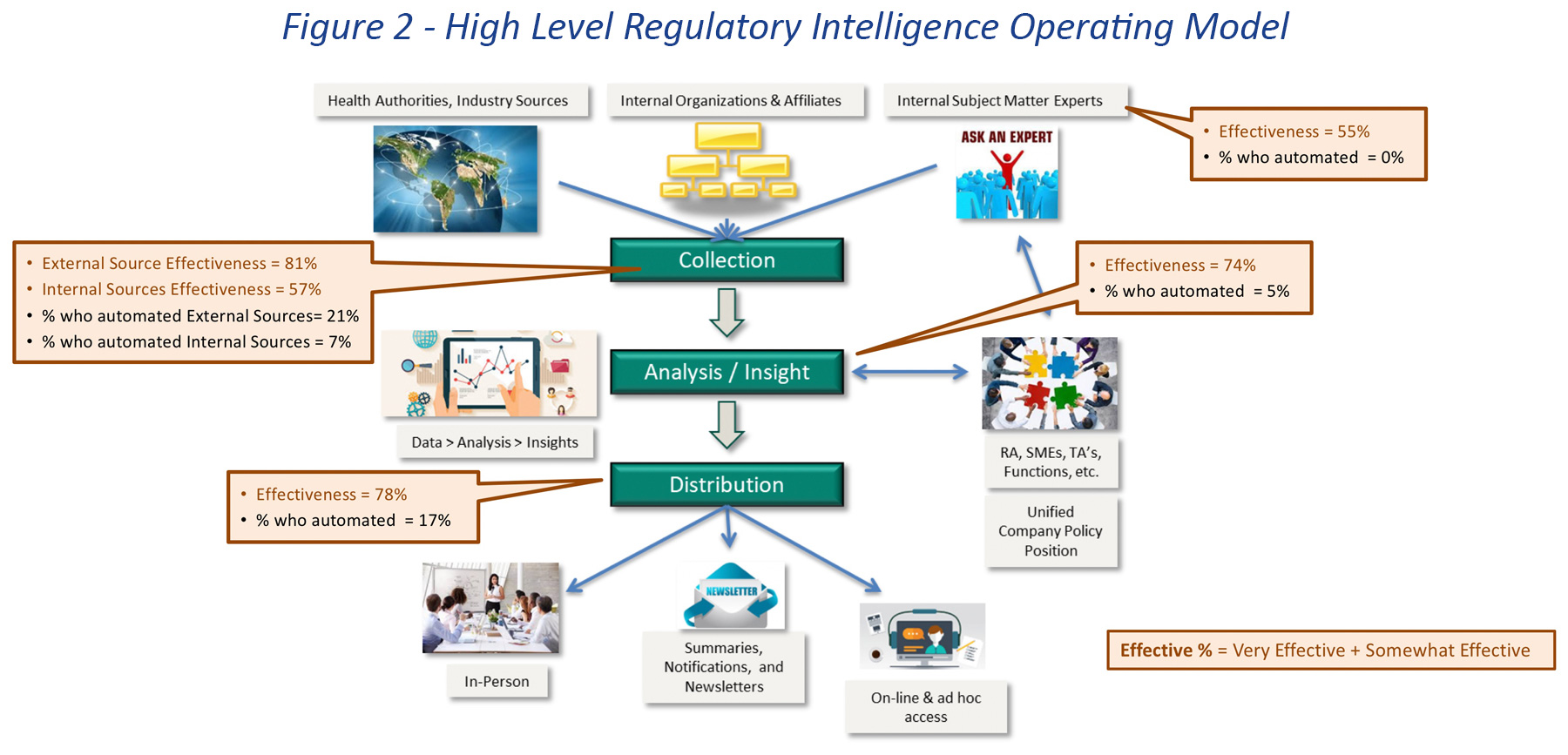
The survey also explored the degree of automation for high level regulatory intelligence activities. The question referred to a typical operating model shown in Figure 2. In this model, the key functions are collection, analysis, and distribution of regulatory information. Contributors and consumers include both internal and external sources and stakeholders.
We found that very few companies have made progress in automating any of these activities. Only two activities, collection from external sources, which includes commercial providers and Health Authorities, and distribution to stakeholders were identified as being automated by a significant number of companies (9 companies and 7 companies, respectively). The use of internal sources is almost entirely manual and an automation target for very few companies.
In his October blog, John Cogan described the challenge of automating complex regulatory use cases from an economic and reward/benefit perspective and found that it was difficult to provide a strong justification at that time.
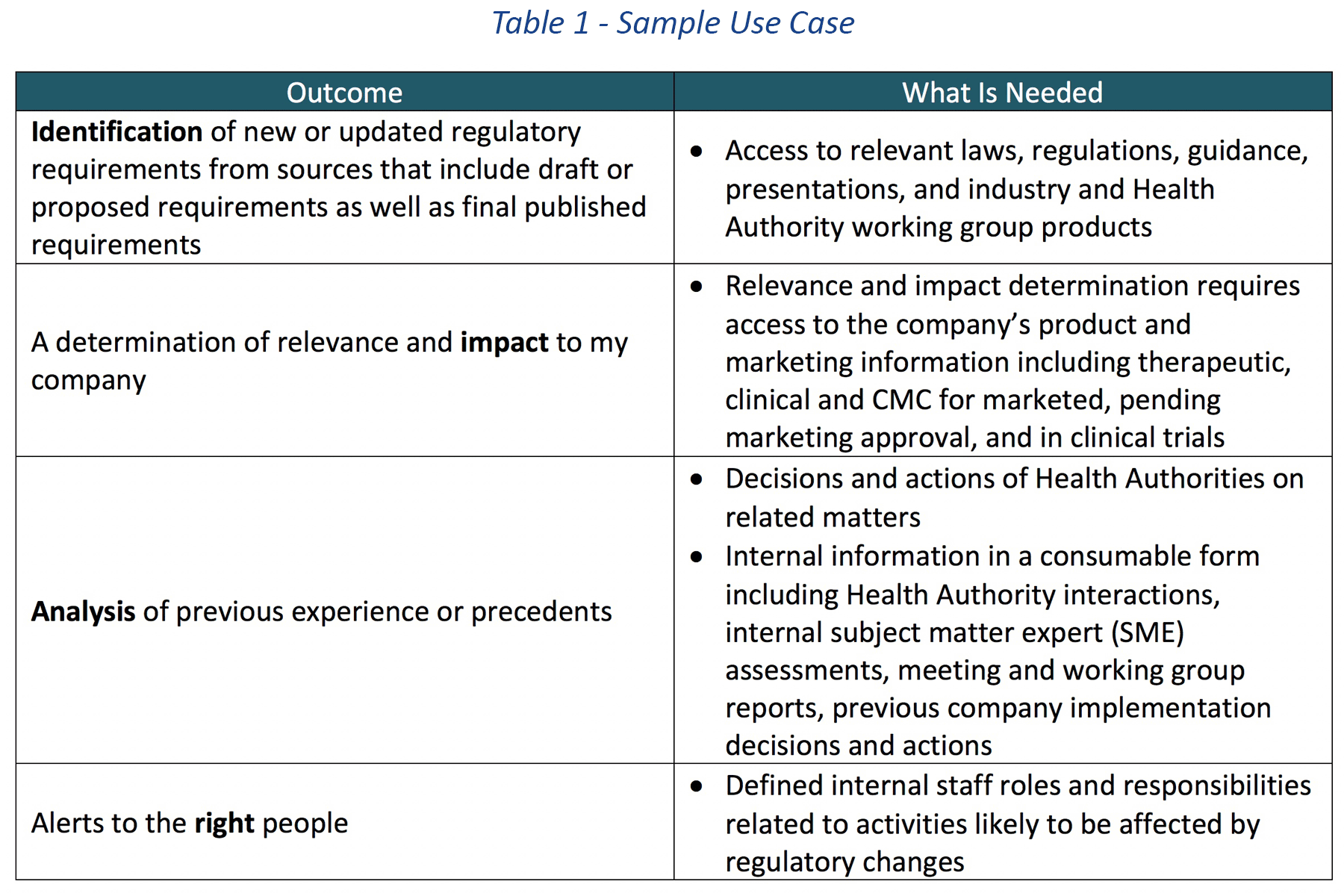
A commonly described regulatory intelligence information analysis use case demonstrates the challenges and complexity in today’s environment.
In this example, the goal is to “automatically” identify and analyze the impact of new or updated regulations from one or more Health Authorities and notify the right stakeholders.
This use case is much more than locating and organizing regulatory requirements, submission formats and the like. There are multiple outcomes implied in this use case that employ aspects of natural language processing and artificial intelligence. For example, Table 1 describes the expected outcomes of the use case and a short list of what would be needed to support automation to achieve each outcome.
In this case, there would be general agreement that the outcomes are highly desirable. And there would likely be general agreement in most companies it is highly unlikely that the items in the “What Is Needed” column are available or could be available as authoritative sources.