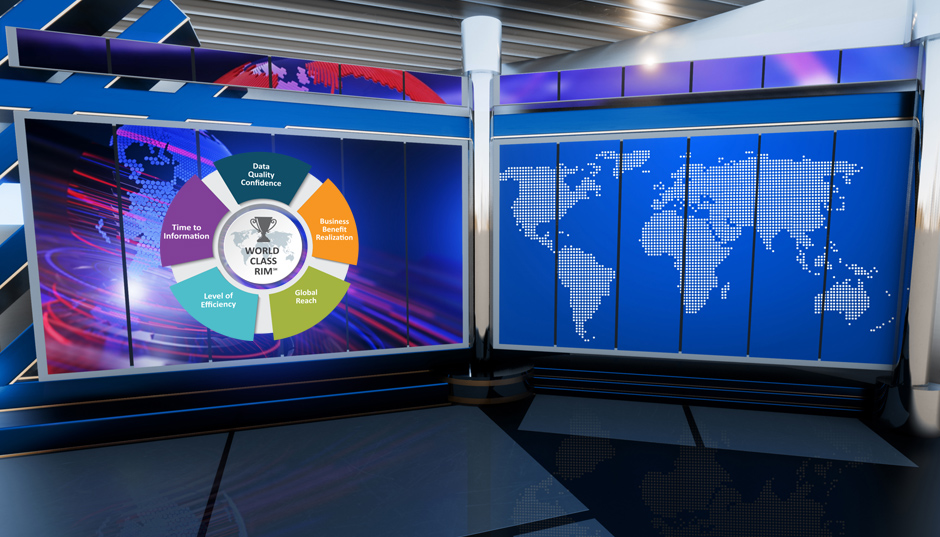
What is World Class RIM℠?
Insights from our Latest Regulatory Information Management Survey
By Steve Gens
As we start our analysis from the 2016 Gens and Associates survey, there are some powerful observations and takeaways that spring to mind. But first, let’s step back and get an overview of the process and industry involvement.
We’ve again broke a record in terms of company participation with 54 taking part in the survey. We’ve always had great participation from larger companies, but this year, we made a concerted effort to bring in smaller organizations and pure medical device companies. We’re very pleased with the demographics, with 30% of respondents coming from large companies, 36% from mid-tier, and 34% from small companies. So we now have a solid understanding of what’s going on with smaller biopharmaceuticals and medical device companies..
In the lead up to developing the survey, we held four survey-design sessions and had 18 companies weigh in on the design. We took the opportunity to have a discussion about world-class regulatory information management (RIM): what is it, what are the important measures, and so on. Through these companies and our own perspectives from our many years in this area, we now have an industry first: a World Class RIM baseline. We believe this will be critically important in helping companies determine their approach. For some years, the industry has had the gold standard for clinical trials and there’s world-class manufacturing, but until now there has been no baseline for defining World Class RIM.
How you measure World Class RIM is important for life sciences companies, and the survey includes a section on measurements and, importantly, business benefits. We know that companies are measuring many different aspects of their RIM capabilities, but so far few have realised the business benefits. The reason for that is the majority are entering their transformation plans, they are in execution mode and hoping to get business benefits in a couple of years.
Common or Disparate
One of the major findings from the survey is that out of original 50 companies participating (the additional four joined the survey a little later), 26 have a common capability, meaning they have consolidated their RIM capability, while 24 companies have disparate capabilities. That could mean having multiple RIM capabilities for different products or different regions: in other words, these 24 haven’t harmonized globally.
Through the survey measurements, we now have empirical proof that having a consolidated RIM capability for all geographies and all products means you are 3.5 times more likely to realize the business benefits, in particular in better process integration, reduced time in submitting to the health authorities, and in higher user productivity.
Those with consolidated RIM solutions have a stronger focus on data quality, and on average their efficiency was 18% greater, for example in product registration, submission production, and submission document management. So those companies are a lot closer to World Class RIM than those with disparate RIM capabilities.
In terms of company size, there’s a fairly even spread of companies using consolidated RIM solutions, the only difference is the larger mid-tiers are doing a lot more transformational change, while the smaller companies are doing incremental change.
We have no doubt that the definition of world-class will evolve and grow as more information is gathered, but having a World Class RIM baseline provides companies with a starting point to measure their own capabilities and assess where they stand against their peers.
Over the coming months we will share more insights from the survey in a series of blogs. In the meantime, we’d welcome your thoughts about World Class RIM and where you are in your journey.