Medical Devices Companies Ahead on Data Standards and MDM, but Lagging on RIM: Gens Survey
By Steve Gens
With the release of our 2016 white paper, Pursuing World Class Regulatory Information Management (RIM); Strategy, Measures and Priorities, I wanted to delve into some interesting findings with regards to medical devices companies.
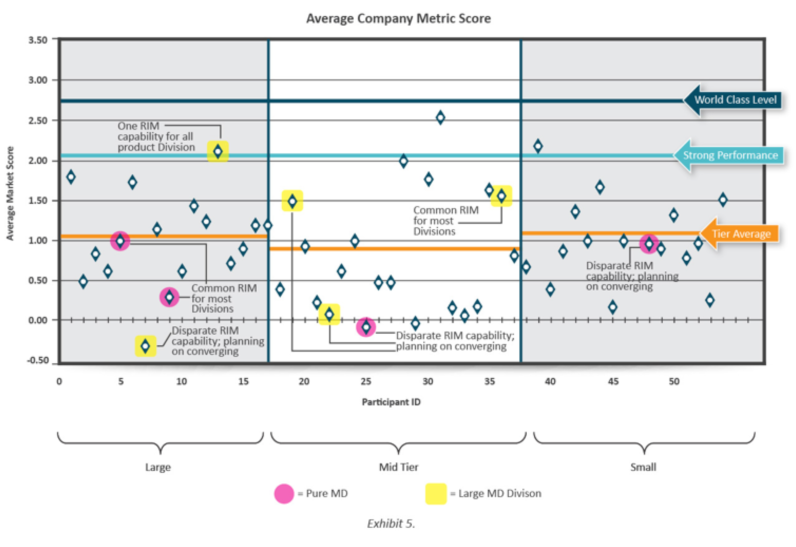
As I’ve discussed, we had 54 companies, a record number of participants in this year’s survey with nine companies that are either pure medical device companies, or ones having a large portfolio of medical device products, meaning more than 2,000 such products.
We’ve analysed and compared their status with regards to regulatory information management (RIM) and made some interesting discoveries showing medical devices companies struggling in many RIM areas but leading the way on data standards and master data management (MDM).
First, and not surprising, medical device companies are lagging about 10 years behind their pharmaceutical and biotechnology counterparts in terms of electronic submission of regulatory information. That’s broadly what we had expected going into the research, largely because the health authorities don’t have clear standards for electronic submissions for medical devices, unlike on the medicinal side which is benefiting from the work done with the ICH 15 years ago on the common technical document (CTD) and electronic CTD or eCTD.
Secondly, there’s also a lot more autonomy at the regional or local level at medical device companies. Whereas see a greater push to tighten the coordination and collaboration between the local affiliate office, regional centres, and headquarters on the medicinal side.
Efficiency Battle
RIM is well-established at most medicinal companies, but medical device organisations are really just starting to invest more in RIM capabilities. So far, they tend to rate their efficiency as low in most areas, and struggle with data quality. Most recognise that a lot of change is needed. Product registration and reporting and analytics are the top two areas where medical devices companies are expecting greatest change, followed by dossier management, the regulatory archive, and health authority correspondence management.
However, the medical devices sector is ahead of medicinal companies in one crucial area: data standards and MDM. Unique Device Identification (UDI), which is used to identify and mark medical devices in the supply chain, has required medical devices to identify products through distribution and use.
With Identification of Medicinal Products (IDMP) looming, pharmaceutical and biotech companies are under pressure to improve data standards and data source capabilities, and many recognise the importance and value of MDM. But for many medicinal companies, the MDM journey is just beginning.
In both the medicinal and medical devices industries, regulatory mandates have been the drivers for improved efficiency. With the pharma industry ahead on RIM and medical devices more advanced on MDM, there are clear opportunities for each industry to learn from the other.
How do the experiences of the respondents to our latest survey resonate with you? Do the trends with RIM and IDMP reflect what’s happening in your company?
Trends at Medical Devices Companies