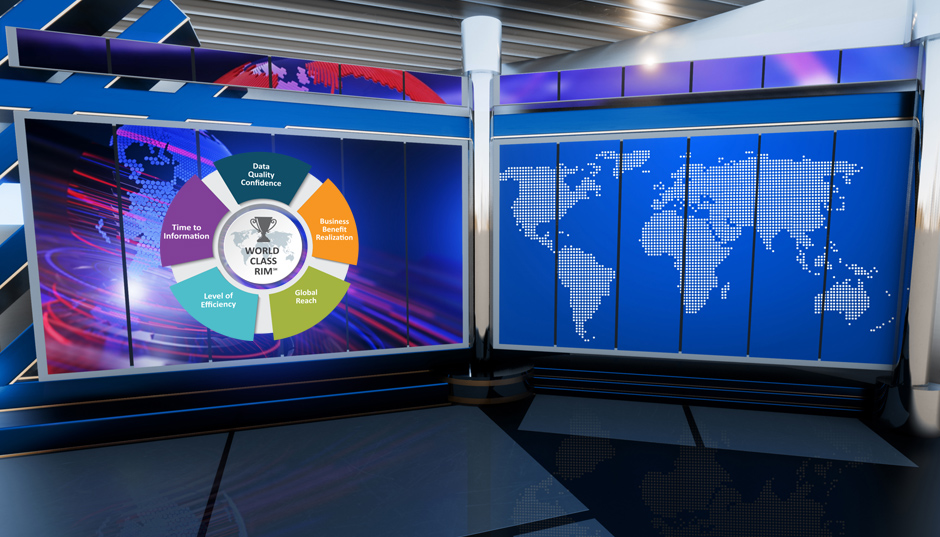
Extra! Extra! Read All About the 2024 WCRIM Study
The 2024 WCRIM Study – Extending the Power of RIM reveals a jump across all performance measurements in our industry standard benchmark since 2022. The data from the current cycle of our research illuminates a clear path to achieving high performance and the degree of performance improvement is driven by consistently applying strong organizational practices, process maturity, and building up data governance.